Draw dipeptide bond Gly-Val. Select the carbon atom of pyruvate that was originally the anomeric carbon aldehyde carbon in the open-chain form of glucose.

Solved Add Coefficients To The Following Reaction Summary To Chegg Com
Glucose a ADP b P.

. The reaction was initiated by the addition of MST and incubation was continued at 37 C for 5. Draw the structure of pyruvate. Two conditions produced crystals of sufficient size for structure determination.
Draw the structure of isoleucine and determine the charge on the molecule in an a. Which compound is likely to predominate in the cell at pH 74. The solid line through the data points represents the fit to a mono-exponential or first order loss.
Which protons come off when. The identity of the metal ion may vary between species of PC. Menu items go here.
B02 with 10 wv PEG 8000 20 vv ethylene glycol 003 M of each halide sodium fluoride sodium bromide sodium iodide in 01 M MESimidazole pH 65 and H02 with 10 wv PEG 8000 20 vv ethylene glycol 02 M each amino acid sodium L-glutamate DL-alanine. A representative plot showing the change in absorbance at 220 nm versus the time after mixing a solution of sodium pyruvate 075 mM with H 2 O 2 10 mM in 40 mM buffer phosphate pH 74 ionic strength 015 at 25C. The activity of dihydrolipoamide dehydrogenase is least sensitive to changes in the ionic strength of the.
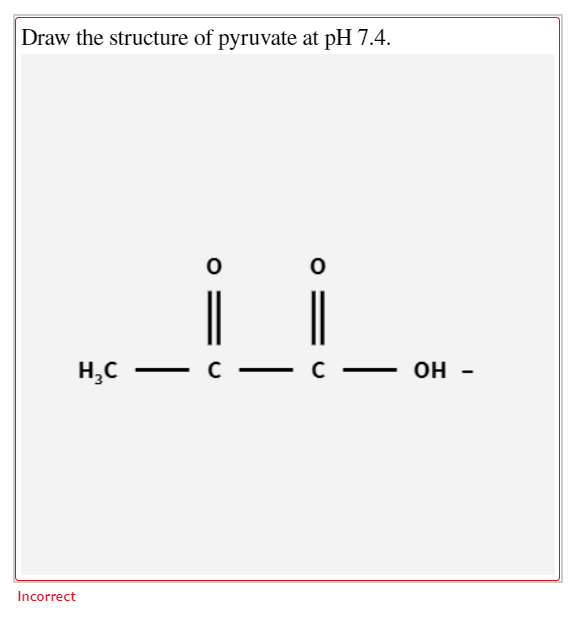
Draw the structure of pyruvate showing its appropriate structure at pH 74. Draw the structure of pyruvate at pH 74. Draw the structure of pyruvate at pH 74.
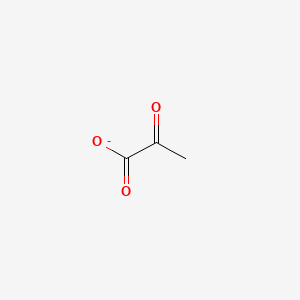
Pyruvate Structure This molecule is the conjugate base of pyruvic acid a three-carbon molecule containing a carboxylic acid group and a ketone functional group. This problem has been solved. Be sure to draw any disulfide bonds that could form.
The structure should have a carbon highlighted in green and bold that corresponds to the original anomeric carbon aldehyde carbon in the openchain form of glucose. Select the structure of pyruvate showing its appropriate structure at pHpH 74. A tool that draws peptide primary structure and calculates theoretical peptide properties.
Look at pKa table for amino acids. Select the structure of pyruvate showing its appropriate structure at pH 74. Basic solution pH 11.
- Want to draw it at pH 5 1 Draw the PEPTIDE BACKBONE fully protonated for as many amino acids as you have. Glucose ADP Pi NAD rar pyruvate ATP NADH Draw the structure of pyruvate showing its appropriate structure at pH 74. Draw the structure of leucine and determine the charge on the molecule in an a.
Amino acid transport detoxification reducing agent steroid hormone metabolism xenobiotic metabolism. Add coefficients to the reaction summary to show the net results of glycolysis. Label the C-terminus of the peptide you drew in 3B above with a Calculate the net charge of LCYRAIDG at pH 15.
Pyruvate C3H3O3- CID 107735 - structure chemical names physical and chemical properties classification patents literature biological activities safety. C NAD x pyruvate y ATP z NADH You do not need to add the water and hydrogen ions necessary to balance the overall reaction. Select the carbon atom of pyruvate that was originally the anomeric carbon aldehyde carbon in the open-chain form of glucose.
Acidic solution pH 1 b. Glucose ADP Pi NAD rar pyruvate ATP NADH Draw the structure of pyruvate showing its appropriate structure at pH 74. Up to 256 cash back If no coefficient is needed leave the answer blank.
Etli a conserved Asp two conserved His residues and a conserved carbamylated Lys in a manner exactly analogous to the metal ion binding site at the active site of 5S. Ala A Arg R Asn N Asp D Cys C Gln Q Glu E Gly G His H Ile I. Neutral solution pH 7 c.
At pH 74 the optimum activity of pyruvate dehydrogenase E1 dihydrolipoamide acetyltransferase E2 and dihydrolipoamide dehydrogenase E3 occur in the ranges of ionic strengths of 006-008 001-002 and 010-015 M respectively. Draw the structure of pyruvate showing its appropriate structure at pH 74. O O HC CC OH Incorrect.
List major functions of glutathione. In PC the metal ion is octahedrally coordinated by pyruvate or a water molecule in the apo structure from R. Briefly 1 ml of the reaction mixture containing 200 m m HEPES buffer pH 74 3-MP 5 m m KCN 25 m m and bovine serum albumin 100 μgml was preincubated at 37 C for 4 min.
A basic solution pH 11 2. Draw the fully protonated structure Q. EH O-NA- Label the N-terminus of the peptide you drew in 3B above with a V.
Up to 256 cash back If no coefficient is needed leave the answer blank. O O HC CC OH. The structure should have a carbon highlighted in green and bold that corresponds to the original anomeric carbon aldehyde carbon in the openchain form of glucose.
The chemical formula for pyruvic acid is C 3 H 4 O 3 and for its deprotonated form is C 3 H 3 O 3. Neutral solution pH 7 c. Draw the structure of the peptide LCYRAIDCG as it would exist at pH 65 under oxidizing conditions.
Draw the structure of pyruvate showing its appropriate structure at pH 74. Acidic solution pH 1 b. Alanine is made from pyruvate transamination of pyruvate with glutamate.

Solved 48 The Structure Of Pyruvic Acid Is Shown Below W Chegg Com
Solved Add Coefficients To The Reaction Summary To Show The Chegg Com

Add Coefficients To The Reaction Summary To Show The Net Results Of Glycolysis Glucose A Adp B Brainly Com

Oneclass Pyruvate Is The End Product Of Glycolysis Its Further Metabolismdepends On The Organism An

Biochemistry Final Exam Part 2 Everything Else Flashcards Practice Test Quizlet

0 comments
Post a Comment